Every year, over 100 million animals are used in lab experiments worldwide science.rspca.org.uk. Yet despite this scale of animal testing, around 90% of drug candidates that seem promising in animals end up failing in human trials cen.acs.org. Enter organ-on-a-chip technology – a cutting-edge alternative that aims to mimic human organs on microchips and dramatically improve drug testing without the need for lab animals. These tiny devices, lined with living human cells, can recreate the key functions of hearts, lungs, livers, and more, offering a more human-relevant testing platform. Regulators and scientists are taking notice: new laws and policies are encouraging non-animal methods, companies are racing to develop organ-on-chip systems, and experts herald this approach as a potential game-changer for medicine and animal welfare. In this report, we’ll explain what organ-on-a-chip technology is, how it works, recent scientific breakthroughs, its benefits over traditional animal testing, the challenges ahead, global regulatory developments, industry activity, and the ethical implications of a future with animal-free drug testing.
What Is Organ-on-a-Chip Technology and How Does It Work?
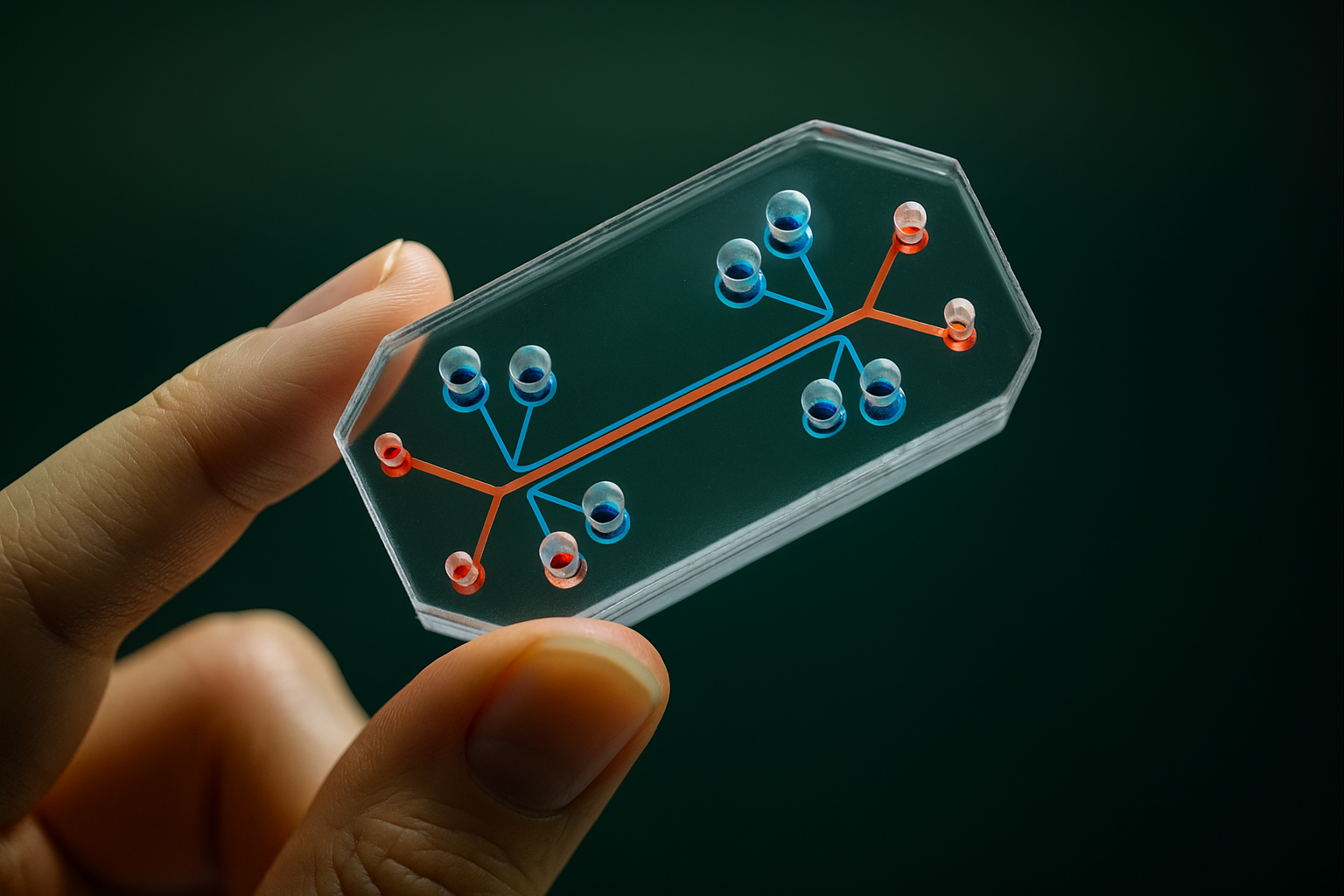
An organ-on-a-chip (OOC) is a miniature device, often about the size of a USB stick or microscope slide, that contains tiny hollow channels lined with living human cells to simulate the functions of a real organ cen.acs.org, clarivate.com. In essence, researchers place human cells (for example, lung cells, liver cells, brain cells, etc.) into a microengineered chamber that provides a 3D environment similar to the human body. These chambers are part of a microfluidic network – tiny channels that continuously flow nutrients, oxygen, and biochemical signals, much like blood flowing through vessels nist.gov. The microchip can also incorporate mechanical forces to imitate organ motions: for instance, a lung-on-a-chip can rhythmically stretch and relax the cell membrane to simulate breathing movements gao.gov.
Organ-on-a-chip devices are not electronic silicon chips, but clear flexible polymers where cells can grow and interact. They create a “miniaturized physiological environment” for cells, meaning the cells experience conditions (fluid flow, nutrition, mechanical stress) similar to those inside an actual human organ nist.gov. Because multiple cell types can be included, an organ chip can replicate complex tissue interfaces. For example, a lung chip might have an alveolar cell layer on one side of a porous membrane and capillary blood vessel cells on the other, allowing interaction just like in a real lung. A liver-on-a-chip might include hepatocytes (liver cells) along with supporting endothelial cells and immune cells (Kupffer cells) to mimic the liver’s microarchitecture clarivate.com. These chips are kept alive in incubators, and sensors or microscopes can monitor how the “mini organ” responds to drugs, chemicals, or disease conditions in real time.
By mimicking a human organ’s microenvironment, organ chips let researchers directly observe human cellular responses without putting a living person or animal at risk nist.gov. In practice, they serve as a bridge between conventional in vitro tests (cells in a dish) and in vivo tests (animals), offering a controlled human-based test system. “It’s called organ-on-a-chip, and it involves growing real tissue from a human organ on a small structure that mimics what that organ tissue would experience inside a body,” explains a report by the U.S. National Institute of Standards and Technology nist.gov. The hope is that these chips will predict how a drug affects human organs more accurately than animal models do. Scientists have already built chips for many individual organs – lung, liver, heart, kidney, intestine, brain, skin, and more – each capturing key aspects of that organ’s biology clarivate.com.
Notably, researchers are also combining multiple organ chips together to simulate larger portions of human physiology. These multi-organ “body-on-a-chip” systems connect the microfluidic blood flow of several organ compartments, so one chip’s output (e.g. liver metabolism of a drug) feeds into another’s input (e.g. effect on heart or kidney) gao.gov. In one groundbreaking demonstration, a team at Columbia University linked four human organ tissues (heart, liver, bone, and skin) on a single chip with a circulating blood-mimicking fluid and immune cells, effectively creating a miniaturized human physiology model engineering.columbia.edu. The entire device was only about the size of a microscope slide, yet kept the tissues alive and communicating for weeks – a major step toward modeling complex, systemic diseases outside the body. “This is a huge achievement for us… now at last we’ve developed this platform that successfully captures the biology of organ interactions in the body,” said the project’s lead, Professor Gordana Vunjak-Novakovic engineering.columbia.edu. Such advances hint at a future where a “human-on-a-chip” could be used to test how a new drug might affect multiple organ systems before any human or animal is ever exposed.
Recent Breakthroughs and Scientific Advances
Organ-on-a-chip technology has rapidly progressed from concept to reality over the past decade, and recent years have seen remarkable breakthroughs. One headline-making advance was the development of multi-organ chips as mentioned above. In 2022, scientists reported the first plug-and-play multi-organ chip with several mature human tissues interlinked by a vascular flow engineering.columbia.edu. This system allowed the different organ tissues to “talk” to each other chemically, just as they do in our bodies. Significantly, all the tissues were derived from the same human stem cells, meaning the chip effectively mimicked a specific patient’s biology – opening the door to truly personalized drug testing in the future engineering.columbia.edu. The ability to maintain multiple organs’ functionality for weeks on a chip is a huge technical leap; it required innovative solutions to give each tissue its own optimal environment while still exchanging signals through a common “bloodstream” on the chip engineering.columbia.edu. This advance garnered attention as it can model complex diseases (like cancer spreading through multiple organs, or heart-liver drug interactions) that single-organ chips alone cannot capture.
Beyond multi-organ integration, researchers have been expanding the capabilities of organ-on-a-chip models in other ways. For example, new chip designs increasingly incorporate sensors and imaging techniques that allow continuous monitoring of tissue responses (such as electrical activity of heart cells or oxygen levels in a lung chip) in real time. There is also a move toward integrating artificial intelligence (AI) and computational models with organ chips. AI algorithms can help design more predictive experiments and analyze the complex data that organ chips produce clarivate.com. A recent article notes that advancements in AI are improving organ-on-a-chip experimental design and data interpretation, hinting that smart algorithms might optimize how we use these chips to foresee drug effects more accurately clarivate.com.
Scientists are also exploring 3D-bioprinting techniques to fabricate organ-on-chip systems with even greater realism blogs.rsc.org. Bioprinting can create three-dimensional tissue structures (like miniature tumors or patches of heart muscle) that are then placed into chips, combining the strengths of tissue engineering with microfluidics. Meanwhile, efforts are underway to achieve standardization across this emerging field so that results are comparable between labs. In early 2024, a NIST-led working group published guidelines to standardize organ-on-a-chip designs and measurements, noting that many groups had been using different protocols and even terminology, which made it hard to compare results nist.gov. By establishing common standards and best practices, the community aims to accelerate development and ensure that organ-chip data are robust enough for widespread use.
Crucially, organ-on-a-chip systems are not just lab curiosities – they’re already yielding scientific insights and outperforming older models in some cases. For instance, studies have shown that organ chips can replicate human-specific drug responses that were missed by animal tests. In one study, a kidney-on-a-chip correctly predicted the kidney toxicity of a drug that had seemed safe in animal trials but later caused harm in humans clarivate.com. Another team using a blood-vessel-on-a-chip was able to detect the tendency of a certain antibody drug to cause dangerous blood clots – a side effect that only turned up in human trials and not in animal tests, but the chip model successfully recapitulated it clarivate.com. These kinds of breakthroughs provide proof-of-concept that organ chips can reveal drug effects that traditional methods overlook. Researchers have developed organ-on-chip models for diseases ranging from lung infections to Alzheimer’s and cancer, enabling experiments on human tissue analogs of these conditions. As one example, brain organoid chips (sometimes called “mini-brains on chips”) are being used to study neurological drug safety: a pharmaceutical study showed that a human mini-brain model could reliably flag neurotoxic side effects of dozens of known drugs cen.acs.org. The rapid advances in such microphysiological systems are giving scientists new tools to explore biology and test treatments in ways that were not possible just a few years ago.
Benefits Over Traditional Animal Testing
Organ-on-a-chip technology offers huge advantages over traditional animal testing, addressing many of the limitations and concerns that have long plagued animal-based research. First and foremost is the issue of human relevance. Because organ chips use actual human cells and recreate aspects of human organ function, their results are often more directly applicable to human patients. By contrast, even the best animal models can differ from humans in critical ways. Drugs that work in mice frequently fail in people, and dangerous side effects might not show up in animals due to species differences. In fact, around 9 out of 10 drug candidates that pass animal tests ultimately fail in human clinical trials for safety or efficacy reasons cen.acs.org. This high failure rate is a strong indication that animal models are imperfect proxies for human biology. “The human brain is incredibly complex… Animals just don’t have a brain that’s anything close to a human’s,” notes Alif Saleh, CEO of an organoid-on-chip company. “The idea that a mouse brain or a rat brain… can predict how a human brain would react to a particular drug – it’s not credible” cen.acs.org. By testing on human-derived tissues in organ chips, researchers can get results that are more predictive of what will happen in actual patients, especially for complex human-specific organs like the brain.
These human-relevant insights have real-world implications for drug safety. Organ chips have already demonstrated an ability to catch toxic effects that animals missed. For example, a human liver-on-a-chip study was able to identify 87% of known drugs that cause liver injury in people cen.acs.org, a performance that significantly improves upon animal test results. Chips can also incorporate patient-specific cells (such as induced pluripotent stem cells from a sick patient), allowing drug responses to be tested on models that reflect genetic and disease idiosyncrasies of actual patient groups. This could reduce the risk of unexpected adverse reactions when a drug enters clinical trials.
Another major benefit is speed and efficiency. Traditional animal tests for drug safety can take years and cost millions of dollars per compound theregreview.org. Maintaining colonies of lab animals, performing lengthy studies, and analyzing the results is a slow and expensive process. Organ-on-a-chip systems, once set up, can often produce data faster and with smaller amounts of a test drug. Automated readouts and high-throughput chip platforms (with many parallel micro-organ assays on a plate) are being developed to screen compounds much more rapidly than using animals. While the technology is still evolving, there is promise that a battery of human-organ chips could someday replace months-long animal studies with faster in vitro tests, saving both time and resources in drug development. A study cited by the FDA showed that computer-based human heart cell models predicted certain cardiac side effects with 89% accuracy, compared to only 75% accuracy in animal tests clarivate.com, highlighting the potential of new approach methods to be not just quicker but more accurate than the animal “gold standard.” As these organ-on-chip models continue to improve, they may greatly reduce the costly late-stage failures of drugs by identifying problematic compounds early in the pipeline.
From an ethical and societal perspective, the reduction in animal use is itself a profound benefit. Each year, innumerable rats, mice, dogs, primates, and other animals are sacrificed in labs, often experiencing pain or distress theregreview.org, science.rspca.org.uk. Replacing even a fraction of this testing with organ-on-a-chip studies means fewer sentient creatures harmed. This aligns with the long-standing “3Rs” principle in science (Replacement, Reduction, Refinement of animal use) clarivate.com. Society increasingly demands cruelty-free testing methods – a trend reflected in consumer pressure and legislation (for example, the EU’s ban on animal-tested cosmetics, and new laws encouraging alternatives in drug testing). Organ-on-a-chip technology directly addresses the ethical call to replace animal experiments with humane alternatives, without compromising on safety. In fact, it promises a win-win: better protection for humans and for animals. Animal testing is also limited by ethical constraints that human-mimicking chips don’t have – researchers can, in theory, push organ chips to explore higher doses or riskier scenarios that could never be ethically done in animals or humans, potentially revealing hazards more comprehensively.
Finally, organ chips can capture aspects of human biology that animal tests often cannot. They allow for direct observation of human cellular responses under a microscope or via sensors, something not possible inside a living animal’s body. Researchers can watch immune cells moving across a chip’s blood vessel wall, or measure real-time release of inflammatory signals from lung cells when exposed to a toxin. This level of detail helps in understanding mechanisms of drug action and disease, providing richer data than the blunt endpoints of many animal tests. Moreover, organ chips can be engineered to represent diverse human populations by using cells from different donors – including those with particular genetic backgrounds or diseases – addressing the issue that animal models don’t reflect human genetic diversity. All these benefits suggest that organ-on-a-chip systems, as they mature, can not only reduce reliance on animals but also usher in a new era of more predictive, humane, and informative drug testing.
Limitations and Challenges
Despite its exciting potential, organ-on-a-chip technology still faces significant challenges and limitations that must be overcome for it to fully deliver on its promises. One immediate challenge is that, as of today, organ chips cannot completely replace animal testing in the drug approval process gao.gov. They are generally used alongside animals and other methods, rather than instead of them. There are several reasons for this. For one, human biology is extraordinarily complex – replicating an entire living organism on a chip is far more complicated than modeling one or two organs in isolation. Most current organ chips focus on a single organ or a small network of tissues. They lack the full systemic interactions present in a whole-body organism (for example, hormonal regulation across organs, or the interplay of the brain with other systems). Even the most advanced multi-organ chips to date include a handful of organ types, which, while impressive, still fall short of a complete human body simulation. As a recent review noted, fully replicating the intricate interactions within a living organism remains exceptionally difficult, and thus the end of animal testing, while a realistic possibility for the future, “may be slow” until these technologies can capture that complexity clarivate.com.
Technical challenges are also significant. Creating a robust, reproducible organ-on-a-chip is not simple – it requires expertise in cell biology, microengineering, and biomaterials. One issue researchers face is obtaining reliable human cells of high quality. Many organ chips use cells derived from stem cells or donor tissues, but these can be variable. Experts estimate that only about 10–20% of sourced human cells are high enough quality for use in organ-chip studies gao.gov. Cells may not survive long or behave normally on the chip, especially if they come from different sources. This makes it hard to ensure consistency. Additionally, standardization is currently lacking in the field. Different labs and companies use different materials, channel designs, cell types, and readout methods for their chips nist.gov. As a result, results from one organ-chip model might not be directly comparable to results from another, even if they nominally represent the same organ. This lack of standardized protocols and benchmarks hinders broader adoption, since drug companies and regulators need confidence that a given chip test is reliable and repeatable. Efforts are underway to address this: in 2023, for example, scientists and regulators convened workshops to define validation criteria for organ-on-a-chip methods and to work toward harmonizing standards globally ema.europa.eu, nist.gov. Establishing reference benchmarks (e.g. how accurately a liver chip must predict known toxins) and qualifying chips for specific “contexts of use” (such as a kidney chip for screening nephrotoxicity) are active areas of work.
Another challenge is scalability and throughput. While some chips are being made in high-volume formats, many organ-on-chip systems are still essentially handcrafted in academic labs or small startups. Producing them at scale with consistent quality, and running many chips in parallel for large studies, is non-trivial. The technology will need to become more user-friendly and industrialized for pharma companies to routinely incorporate it. Automated fluid handling, imaging, and data analysis for chip experiments are still being refined. Cost can also be a limiting factor: currently, setting up organ-on-chip assays may be more expensive and time-consuming than certain simpler lab tests. The U.S. Government Accountability Office notes that some organ-on-chip research costs more and takes longer than traditional animal or cell culture studies, at least in these early stages gao.gov. Over time, costs may come down with better manufacturing and wider use, but for now budget constraints mean chips are used selectively.
Data interpretation and validation present further hurdles. Regulators and industry scientists need to be convinced that organ-on-chip results accurately correlate with human outcomes. This requires extensive validation studies comparing chip predictions to real clinical data and to animal studies. As of now, the field is still gathering that evidence. A GAO report highlighted that a lack of well-documented benchmarks and validation studies makes it hard for end-users to know how much confidence to place in a given organ chip’s results gao.gov. For instance, if a liver-on-a-chip says a drug is safe, how sure can we be that it won’t cause liver damage in humans? Building that trust will require time and multiple studies. Companies can also be hesitant to share data openly – often for competitive or intellectual property reasons – which slows collective learning gao.gov. Increased data sharing and collaboration, perhaps through consortia or public-private partnerships, would help the field mature faster.
Finally, there are regulatory uncertainties. Because organ-on-a-chip is a novel technology, many regulators are still becoming familiar with it. Guidelines on how to use chip data in drug applications are only now being formulated. The FDA and other agencies have historically relied on animal data, and changing those entrenched practices involves careful deliberation. As of early 2025, experts reported that regulators had a “lower level of familiarity with OOCs than other methods” and that guidance from agencies could be clearer gao.gov. This is beginning to change (as we’ll discuss in the next section), but until formal frameworks are established, some drug developers may be reluctant to invest heavily in organ chips without knowing how regulators will view the data. In summary, while organ-on-a-chip systems hold tremendous promise, they are not a magic bullet just yet. Significant scientific and practical challenges remain in making them robust, trusted, and widely usable. Overcoming these challenges will require continued R&D, investment, and close collaboration between scientists, industry, and regulators – but progress is well underway.
Global Regulatory Developments
Regulatory agencies around the world are recognizing the potential of organ-on-a-chip and related non-animal testing methods, and they have begun updating policies to accommodate and encourage these innovations. In the United States, a landmark change came with the passage of the FDA Modernization Act 2.0 in late 2022. This bipartisan law removed a decades-old requirement that all new drug candidates must be tested on animals before entering human trials clarivate.com. In other words, the U.S. Food and Drug Administration (FDA) can now accept alternative preclinical testing data, including data from in vitro models like organ-on-a-chip, instead of strictly requiring animal studies. This was a huge victory for advocates of animal-free research, who had long argued that antiquated regulations were preventing the use of superior modern methods. As an FDA spokesperson noted, the agency can now clear drugs for human trials using “nonclinical tests” such as organ chips, organoids, computer models, and other approaches, rather than relying solely on live animal data emulatebio.com, pubmed.ncbi.nlm.nih.gov. However, passing a law is just the first step – implementing this flexibility in practice is a gradual process.
Fast forward to 2025, and the FDA has signaled even stronger support for moving away from animal testing. In April 2025, the FDA announced a bold roadmap to phase out many animal tests within the next 3–5 years cen.acs.org. The agency stated its goal is to make animal studies the “exception rather than the norm” for evaluating drug safety, starting with certain product areas like monoclonal antibody drugs and expanding to all drug types cen.acs.org. The FDA even suggested it might offer fast-track review for drug submissions that use validated alternative methods in place of animals cen.acs.org. Industry observers have described this as a watershed moment. “It feels like a key watershed, historic moment,” said Dr. Tomasz Kostrzewski, chief scientific officer of CN Bio, a UK-based organ-on-chip firm, regarding the FDA’s new plan. “This is the point where the FDA is saying, ‘We’re totally committed to move forward and away from animals in a 3–5-year window.’” cen.acs.org. This clear and deliberate shift in policy has energized the organ-on-chip industry – companies reported immediate upticks in interest from investors and pharma clients after the FDA’s announcement cen.acs.org.
On the other side of the Atlantic, Europe is also moving to integrate organ-on-a-chip into the regulatory framework. In September 2021, the European Parliament passed a resolution calling for an EU-wide action plan to accelerate the transition to innovation without the use of animals ema.europa.eu. This political push has spurred European regulators to act. The European Medicines Agency (EMA) formed a dedicated 3Rs Working Party, which in 2023 initiated efforts to qualify and validate microphysiological systems (including organ-on-chip) for regulatory use ema.europa.eu. The EMA’s workplan includes organizing workshops with industry and academia, defining regulatory acceptance criteria for organ-on-chip tests in specific contexts (for example, using a liver chip for drug toxicity assessment), and even collaborating internationally to harmonize these criteria ema.europa.eu. In fact, regulators from the U.S., Europe, and other regions have set up a “worldwide cluster” to coordinate on new approach methods and to share knowledge on how to evaluate them ema.europa.eu. This global harmonization is important – it means agencies are talking to each other to ensure that, say, a test method accepted by the FDA might also be accepted by the EMA or Japan’s authorities, and vice versa.
Europe has also supported alternative testing through institutions like the EU Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM), which has been researching and validating non-animal methods for years clarivate.com. The momentum from the political side (European Parliament) and scientific side (EMA and ECVAM) suggests that Europe is laying the groundwork for eventually approving drug safety data from organ-on-a-chip models. While as of 2025 no major regulator has completely eliminated animal tests, the direction is clearly towards a future where organ chips and other non-animal assays play a central role in safety evaluations.
Concrete examples of regulators embracing organ-on-a-chip are beginning to emerge. In 2024, the biotech company Argenx included data from a MIMETAS liver-on-a-chip model as part of an Investigational New Drug (IND) application to the FDA – reportedly one of the first times organ-on-a-chip data supported an official drug filing mimetas.com. The organ-on-chip tests helped demonstrate the safety profile of Argenx’s new drug in a human-relevant system, and this was accepted by regulators as supplemental evidence. MIMETAS’s CEO, Jos Joore, highlighted the significance: “By embracing advanced human in vitro models over traditional methods like 2D cell culture and animal models, we can bridge a critical gap towards advancing new therapies.” mimetas.com This case exemplifies how regulatory changes (like the FDA Modernization Act) are translating into real-world applications, with companies confident enough to submit organ-on-chip results in their approval packets.
In the coming years, we can expect more formal guidelines to be issued. The FDA has its Advancing Alternative Methods initiative, which provides resources and funding to develop and qualify methods like organ chips clarivate.com. The EMA, as noted, is working on guidance documents. Regulatory science agencies are also funding research to directly compare animal studies with organ-on-chip outcomes, to build the evidence base needed for wider acceptance. It’s worth noting that regulators will likely take a cautious approach: early use of organ chips might be as adjuncts to animal data (to provide additional insight or to reduce the number of animals needed, rather than replacing them outright). But if these methods continue to prove their merit, it’s conceivable that for certain tests – e.g. liver toxicity or skin irritation – an organ-on-a-chip could become an officially recognized replacement for an animal test. The trajectory is set: globally, the regulatory landscape is shifting to welcome innovative drug-testing methods that don’t rely on animals. The 2020s are shaping up to be the decade when organ-on-a-chip moves from the lab bench to an accepted part of the drug approval process.
Commercial Players and Market Activity
With growing scientific validation and regulatory support, the organ-on-a-chip field has seen a surge of activity from innovative startups, academic spin-offs, and even established companies. A small but rapidly expanding industry has formed around designing and supplying these “organ-on-chip” platforms to pharmaceutical and research organizations. Perhaps the most well-known player is Emulate, Inc., a Boston-based company that emerged from Harvard’s Wyss Institute (the group that pioneered the lung-on-a-chip). Emulate produces a line of organ chips (liver, intestine, lung, brain, etc.) and has been at the forefront of commercializing this technology. According to Emulate’s CEO, interest in their organ chips has spiked recently – after the FDA announced its plan to curb animal testing, Emulate was “fielding requests from potential clients” and even hearing from investors eager to put more money into the company cen.acs.org. It’s a clear sign that the market expects demand for organ-on-chip solutions to grow as pharma shifts its development strategies.
Emulate is not alone; several other companies are making waves. CN Bio, a UK-based firm, offers organ-on-chip systems and has developed a multi-organ platform (often called a “microphysiological system”) that can link liver with other organ modules. CN Bio has been active in partnerships and in publishing validation studies of their liver chips for toxicity testing. MIMETAS, based in the Netherlands, is another leader – known for its OrganoPlate® technology, which is essentially a microfluidic plate containing many miniature organ models for high-throughput screening. MIMETAS has secured collaborations with major pharma companies; for example, it entered a strategic partnership with Astellas Pharma in 2023 to use organ-on-chip models for cancer drug research mimetas.com. Mimetas also worked with the biotech Argenx, as mentioned, providing organ-chip data for an IND submission – a milestone that demonstrates the commercial relevance of its platform mimetas.com.
In the United States, Hesperos, Inc. (a Florida-based startup co-founded by pioneering researcher Michael Shuler) focuses on multi-organ systems and offers testing services using its “human-on-a-chip” models. Hesperos has reportedly collaborated with big pharma companies like Sanofi, AstraZeneca, and Apellis to screen drug candidates for safety and efficacy using its multi-organ chips cen.acs.org. These partnerships with household-name pharmaceutical firms indicate that even large companies are evaluating organ-on-chip data alongside traditional studies. Another notable U.S. company is AxoSim, which specializes in nerve and brain models (such as “mini-brains” and nerve-on-chip platforms) for testing neurological effects; they too have attracted biotech clients interested in assessing neurotoxicity without animal models cen.acs.org.
The organ-on-a-chip sector also includes companies like TissUse (Germany), which offers a “multi-organ bioreactor” platform, and Nortis (USA), known for its microfluidic vascular chips. Even large contract research organizations (CROs) such as Charles River Laboratories have started investing in organ-on-chip technology or partnering with organ-chip companies criver.com (as they foresee clients requesting these assays). In short, an ecosystem of producers, service providers, and collaborators is taking shape.
The market trajectory for organ-on-a-chip is very promising. While still relatively small in dollar terms today, it is growing at a rapid pace. Market research reports estimate the global organ-on-a-chip market was on the order of only ~$150 million in the early 2020s, but project explosive growth (30–40% annually) in the coming years grandviewresearch.com. Some forecasts expect the market to reach nearly $1 billion by the end of this decade grandviewresearch.com, driven by increasing adoption in drug discovery, toxicology testing, and academic research. This growth is fueled not just by pharma demand, but also by funding from government initiatives and research grants aiming to improve testing methods. For instance, agencies like the U.S. NIH have funded “Tissue Chip” programs to develop organ-on-chip models for diseases and even sent some of these chips to the International Space Station for experimentation in microgravity (expanding the range of applications for the technology).
Investor interest in organ-on-a-chip startups has followed suit. Venture capital and corporate investors see the potential for these technologies to revolutionize parts of the $180+ billion preclinical research market. Emulate, for example, has raised significant funding and inked deals to supply chips for testing drug safety (one partnership involved Moderna, using Emulate’s liver-on-a-chip to screen the safety of lipid nanoparticles used in mRNA vaccine delivery) cen.acs.org. As regulations increasingly favor non-animal data, pharma companies may pour more resources into organ-chip testing to stay ahead of the curve, further boosting the market.
Of course, with opportunity comes competition and some growing pains. Companies must prove that their specific organ chip models are reliable and scientifically valid. They often work closely with regulatory agencies to qualify their devices. There have been reports of smaller organ-on-chip companies facing funding hurdles, especially if reliant on government contracts that can fluctuate cen.acs.org. However, the overall trend is that commercial activity is intensifying. The space is also seeing a convergence of disciplines – biotech firms are hiring microengineers, software experts, and biologists alike to refine these products. As more success stories emerge (such as a drug developed with the help of organ chips making it to market), it will further validate the business case for this technology. In summary, the organ-on-a-chip industry is moving from a niche, pioneering phase into a more mature phase of scaling and integration into mainstream drug development, backed by a favorable regulatory and societal tailwind.
Ethical and Societal Implications
The rise of organ-on-a-chip technology carries profound ethical and societal implications, mostly very positive, but also with some considerations for how we conduct biomedical research. On the ethical front, the most obvious benefit is the potential to greatly reduce (and eventually eliminate) the use of animals in drug testing and research. This addresses a longstanding ethical issue: traditional drug testing has required sacrificing countless animals, raising concerns about animal welfare. Replacing those tests with human-cell based chips means far fewer animals would be subjected to experimentation. Animal welfare organizations have hailed this trend – when the FDA announced its move away from animal tests, animal rights groups were among the loudest voices celebrating cen.acs.org. The public, too, is increasingly concerned with how products are tested. Surveys show consumers prefer ethically sourced products and have pressured legislators to act on animal testing theregreview.org. The shift toward organ-on-a-chip is in part a response to this societal demand for cruelty-free innovation. It offers a tangible solution to the question, “If not animals, then how?” – demonstrating that we can uphold safety and scientific rigor without harming animals.
Another ethical dimension is the fairness and human relevance of research. We often forget that reliance on animal models isn’t just risky for humans, it can also be unfair to patients if it delays or misleads drug development. For example, if a cure for a human disease fails in animals and is shelved, humanity loses out due to another species’ biology not matching ours. Conversely, an unsafe drug might pass animal tests and go on to hurt human volunteers in clinical trials. Organ-on-a-chip addresses this by focusing on human biology from the start, potentially leading to safer trials and fewer tragedies. By providing more predictive data, it can spare human volunteers from exposure to drugs that would have failed anyway. In this sense, organ chips benefit society by improving the safety of clinical research – fewer trial participants put at risk – and by possibly speeding up the development of cures (since ineffective compounds can be weeded out earlier, and promising ones identified with more confidence).
The transition to organ-on-a-chip and similar methods also has implications for the scientific community and workforce. As animal testing becomes less central, researchers will need new skills (for instance, tissue engineering, microfluidics, and computational analysis) to use and develop these advanced in vitro systems. There may be a cultural shift in labs and education: future toxicologists and pharmacologists could train on human-mimicking chips instead of learning surgery on lab animals. This could foster a more human-focused mindset in research from the outset. Ethically, many young scientists are enthusiastic about techniques that don’t require harming animals, so organ chips can make biomedical careers more attractive to those who object to animal use. That said, care must be taken to manage the transition for those whose livelihoods currently depend on animal-based research (such as breeders of lab animals or certain lab technicians). Over time, resources can be redirected – for example, facilities that once housed animals could be converted to tissue culture labs. The hope is that scientific progress will go hand-in-hand with ethical progress, and organ-on-a-chip provides a path for that.
There are also broader societal questions to consider. If organ-on-a-chip and related technologies (like organoids and computer models) become the norm, society will need to ensure regulatory and legal frameworks are updated to keep pace. For instance, how do we set accountability if a drug is approved based on a new method that later shows unanticipated effects? Ensuring that organ-on-chip methods are properly validated helps mitigate this. Some ethicists argue that as we adopt human-based models, we must also revisit how we define safety and efficacy standards – possibly raising them, since we’ll have more precise tools. On a global scale, equitable access to these technologies is a consideration: developing countries might lack the resources to implement high-tech organ chip testing quickly, so there could be a need for international support or technology transfer, otherwise a gap might arise where only certain countries move away from animal testing initially.
From a societal values perspective, the move towards animal-free testing reflects a growing compassion and respect for other living creatures. It resonates with the idea that scientific advancement should not come at the cost of unnecessary suffering. If successful, organ-on-a-chip technology could become a point of public pride and support, much like the space race or other grand scientific endeavors, because it solves a moral dilemma while advancing science. We might see a future where medical breakthroughs are lauded not just for saving human lives but also for not taking animal lives in the process. Already, we see language in policy circles framing the reduction of animal testing as a mark of progress and innovation ema.europa.eu.
In conclusion, the ethical and societal implications of organ-on-a-chip technology are largely transformative and positive. It offers a future where we innovate more humanely, aligning scientific practices with society’s evolving moral expectations. Of course, transparency and education will be key – the public should be made aware of these new methods and assured of their effectiveness, to maintain trust in how medicines are tested. If organ-on-a-chip fulfills its promise, we may look back on animal testing as a crude, archaic approach akin to other outdated practices in medicine’s history. The journey isn’t over, but each advancement in organ-on-a-chip brings us a step closer to a world where life-saving drugs can be developed without sacrificing lab animals, to the benefit of both humans and animals alike.
Expert Insights and Future Outlook
Many experts in the fields of pharmacology, bioengineering, and ethics are optimistic that organ-on-a-chip technology will play a central role in the future of drug development. Dr. Donald Ingber, the Harvard professor who led the development of the first lung-on-a-chip, often points out that these systems can “bridge the gap” between petri-dish experiments and living humans in a way nothing else can. He and others emphasize that organ chips provide human context to experiments – something animal models will inherently lack. As more validation data emerge, confidence in these systems is growing. Industry leaders like Jim Corbett of Emulate highlight how quickly things are changing: “This is a clear and deliberate shift,” Corbett said of the FDA’s new stance, underscoring that what was once a futuristic idea is now being actively integrated into regulatory science cen.acs.org.
At the same time, experts caution that we must be realistic and rigorous. No single method will solve all problems, and organ-on-a-chip is not a panacea. Dr. Anthony Holmes of NC3Rs in the UK has noted that a combination of methods – organ chips, computer modeling, high-throughput cell assays – will collectively replace the animal tests, and that collaboration is key. This sentiment is echoed by regulators who are engaging stakeholders through workshops and working groups nist.gov. The future they envision is one of “new approach methodologies” working in concert to improve predictions. In that future, organ-on-a-chip is seen as a cornerstone technology that can simulate human organ responses, while other tools (like computational models) can simulate systemic physiology or genetics. Together, these could render animal tests obsolete.
One striking insight from industry came from the CEO of Mimetas, who commented on an IND filing supported by their organ-on-chip data: embracing human-relevant models early can accelerate therapy development mimetas.com. This reflects a broader shift in mindset – using human biology as the default testbed, rather than relying on cross-species extrapolation. The expectation is that as more success stories appear (such as drugs whose dangerous side effect was caught by a chip, or a therapy developed quickly thanks to chips), the entire pharmaceutical paradigm will shift to “human-first” testing models. Companies that adapt to this will likely have a competitive edge, being able to fail fast (eliminate bad drugs sooner) and focus on promising candidates.
Looking ahead, experts predict some fascinating developments. Personalized medicine could be supercharged by organ-on-a-chip: imagine taking cells from a patient with a particular cancer, growing a micro-tumor on a chip along with that patient’s own immune cells, and then testing a panel of drugs to see which works best – all before treating the patient. This could become a reality and would tailor treatments to individuals with unprecedented precision. Researchers are also looking at integrating CRISPR gene-editing with organ chips to model genetic diseases on-chip and test gene therapies. Another area is environmental and chemical testing – regulatory agencies responsible for chemical safety (not just drugs) are interested in organ chips to test cosmetics, food additives, or industrial chemicals for toxicity without animal tests. The EPA in the U.S., for example, has initiatives to reduce animal testing for chemicals by 2035, and organ chips are likely to be part of that solution.
In summary, the expert consensus is that organ-on-a-chip technology is poised to revolutionize how we approach drug testing and disease research, but it will require continued effort to realize its full potential. The optimism is coupled with a sense of responsibility: to thoroughly validate these systems, to ensure they are accessible and used correctly, and to share knowledge widely. As this field matures, the once far-fetched idea of drug development without animal testing is coming into focus. Each tiny microfluidic chip, with its living human cells, represents both a scientific breakthrough and an ethical advancement. Together, they are steering us toward a future of safer, faster, and more humane drug discovery – a future where lab rats, rabbits, and monkeys are no longer the default subjects of testing, and where human biology on a chip leads the way in saving human lives.
Sources:
- Ingber, D. et al., Wyss Institute, Harvard – Human Organs-on-Chips Overview cen.acs.org
- U.S. GAO – Human Organ-on-a-Chip: Benefits Over Animal Testing, Challenges to Adoption (May 2025) gao.gov
- Walrath, R., Chemical & Engineering News (May 2025) – “FDA’s shift from animal testing opens doors for organoid makers” cen.acs.org
- Lake, D., Lab on a Chip Blog (RSC) – “Breakthrough technologies in Organ-on-a-Chip” (Jul 2024) blogs.rsc.org
- Clarivate Analytics – “Beyond animal testing: the rise of organs-on-chips” (Oct 2024) b clarivate.com
- NIST News – “Developing Standards for Organ-on-a-Chip Research” (Feb 2024) nist.govnist.gov
- EMA 3Rs Working Party Report (2023) – Organ-on-Chip qualification for regulatory use ema.europa.eu
- Columbia Engineering News – “Plug-and-Play Organ-on-a-Chip” (Apr 2022) engineering.columbia.edu
- Mimetas Press Release – Organ-on-Chip data in FDA IND application (Jul 2024) mimetas.com
- RSPCA Science – Animals in research statistics science.rspca.org.uk
- The Regulatory Review (Penn Law) – “Is It Time to End Animal Testing?” (Jan 2024) theregreview.org
- C&EN / Biospace – Animal testing market and failure rates cen.acs.org